It’s well-known that patient recruitment in clinical trials can be difficult, but keeping participants throughout the study can be just as demanding for clinical research teams. With many clinical trials taking place over a prolonged period of time, there is always the potential for drop-outs to occur, sometimes jeopardizing the entire study.
The issues that can be caused by low participant retention make having a retention strategy in place an integral part of the clinical trial process. Thankfully, there are several ways research teams can improve patient retention in a clinical trial, ensuring successful outcomes for the entire study.

Struggling to recruit participants for your clinical trial?
Discover how our expert recruitment strategies can speed up your trial's enrollment process, ensuring you meet your participant goals on time.
What is patient retention in clinical trials?
Patient retention in a clinical trial is defined by the NIH as “the strategy and tactics designed to keep participants enrolled in clinical trials from discontinuing participation and dropping out”. As one of the largest challenges facing clinical trial sites, sponsors, and CROs, patient retention is highly important.
Of course, good recruitment before a trial begins is paramount, but even with an exceptional recruitment strategy in place, participant dropouts can still happen. The research team should make sure retention rates are closely monitored so that they can quickly take steps to prevent further losses and keep the trial on track.
Why is patient retention in clinical trials important?
Patient retention in clinical trials is highly important as it affects three main components of a clinical study’s success: study validity, time, and money. If large numbers of participants drop out of a trial, it can be delayed significantly, sometimes by years.
Besides missing deadlines and extending a trial past its proposed timeline, money is an important consideration. If a study needs to be prolonged due to retention issues, this could cost sponsors thousands, and sometimes millions of dollars.
Poor retention in clinical trials can threaten the study’s validity by leading to incomplete data before the trial is finished. In some cases, researchers might attempt to continue with fewer participants, but this can weaken the trial’s accuracy and reliability, resulting in subpar outcomes. Simply put, keeping participants engaged is essential for the success of a clinical trial, making it vital to have strategies in place to address potential retention issues.
What are the most common reasons for participant dropout?
There are several reasons why participants drop out of clinical trials, from financial burdens to difficulties with the location. Understanding the main reasons for patient dropout is critical to establishing strategies that mitigate these challenges.
Looking to improve clinical trial recruitment? Discover proven strategies to enhance enrollment and promote diversity in your studies. Learn how to make a greater impact today in our detailed guide!
Inconvenience and overwhelm
Participating in clinical trials often requires a significant time and effort commitment, which can sometimes feel like a burden. This is especially true for patients who have serious health issues. However, it’s important to remember that patients who feel like the trial has become a burden can choose to stop taking part at any time.
Logistics can also play a role in making clinical trials feel inconvenient, especially for participants who struggle with long distances, frequent visits, or high costs. Many trials are held in urban centers, making it harder for rural participants to access them. Using virtual tools or other convenient methods can help bridge this gap. Without these solutions, rural patients may feel neglected, which can lead to lower retention rates.
Financial barriers
Choosing to take part in a clinical trial often comes with a financial burden, even with support from the research team. While most costs for travel are reimbursable, travel to and from research sites can get expensive, especially as participants often need to pay from their own pocket first. This financial burden may become too much for some people, leading to dropouts.
Feeling underappreciated
Dropout rates can increase in participants who do not feel valued during a clinical trial. This can happen if patients no longer feel they are making a meaningful contribution to medical research, or that they are not receiving any value in exchange for their time and effort. Having enough participants involved is key to a successful trial, which means researchers need to ensure participants continually feel valued and supported.
No change in the condition state
Participants taking part in a study for a specific condition may feel discouraged if they do not experience any progress or change in their health. Likewise, if participants are placed in a control group, whether knowingly or not, they are unlikely to show any improvement and are more likely to drop out. Patients should be regularly informed of the importance of their continued participation, regardless of how their condition progresses.
Unclear expectations
At the beginning of a clinical trial, and throughout the study, participants should be continually informed and reminded of what is expected of them. This ensures they know what to expect and can plan accordingly, without hidden surprises. Without clear guidance and expectation setting, participants may feel discouraged and more likely to leave the study. Researchers must make sure they clearly communicate and improve communication methods if retention rates start to decrease.
Personal matters
In some cases, participant dropout is unavoidable, such as when personal or family matters occur. Family problems can crop up at any time, and it’s difficult for researchers to plan for and mitigate against them. However, being aware that these issues can happen is a good first step.
Fitting participation in a trial around work schedules and family commitments can be difficult. For example, participants with young children may find it harder to maintain their time commitment. Participants may also be unable to take time off work to attend the trial site when required.
Patient relocation
During the course of a study, a patient may need to relocate and therefore may not be able to access the same study site. This is a common issue that cannot be rectified reactively, and researchers should make every effort to filter this issue out during the recruitment stage. Of course, unplanned relocations may still occur.
Adverse events or fear
Humans naturally fear the unknown, and adverse effects could make patients more fearful of the rest of the trial. Similarly, interventions or procedures that make patients feel uncomfortable, cause pain, or are scary, may further increase the likelihood of patients leaving the trial.
As clinical trials study the unknown in many cases, participants may have concerns about potential side effects or the safety of the study. There may be side effects or adverse events that occur or are thought to occur, from the provided intervention, which can evoke fear. Whether these effects are due to the treatment or not, participants who experience them are at a higher risk of dropping out of the study.
How to improve retention in clinical trials
Improving patient retention in clinical trials should be a priority for researchers. Successful retention can help the trial stay on schedule, saving both time and money. The key way to improve participant retention is person-centered care. Research teams must focus on patients and their needs, not just what they need to do to adhere to participation guidance. Participants should have ample opportunity to offer feedback, share their experiences, and feel heard by researchers.
Trial sponsors and researchers should also concentrate their efforts on technology, as this can be a huge help in streamlining processes that save time and money. There are a number of other strategies researchers can use to improve retention in clinical trials.
Reduce the burden of participation
Reducing the burden of participation is key to retaining patients in a clinical trial. It’s important for researchers to simplify the study protocols as much as is feasible to make participation more convenient. They can do this by reducing the frequency of site visits, simplifying procedures, and streamlining data collection.
Additionally, researchers should look to remote or decentralized trial methods where telemedicine, home visits, or mobile health technologies can be used. With these elements included in a trial, participants can complete some study activities from home, reducing the need for travel and ensuring more convenience.
Flexible scheduling should also be a consideration, with flexible appointment times in the evening and at weekends that better accommodate the work and personal schedules of patients. When designing a trial, research teams could also try to make trials more convenient by choosing study sites closer to participants or using multiple locations to minimize travel time.
Accommodate life changes
Understanding that participants' life changes are not always avoidable is key to improving retention. Researchers can support these changes by using adaptive study designs that allow for changes in the study protocol with options like virtual follow-ups, offering relocation support to continue participation at another site, and maintaining regular contact to stay informed about participants'changing needs.
Mitigate financial costs
One of the most common reasons for patient dropout is the financial burden on participants, but thankfully, this is a fairly easy fix for researchers. Travel, parking, and other out-of-pocket expenses should be fully reimbursed promptly, or prepaid cards could be offered to cover these costs upfront.
Additionally, researchers may also consider time compensation for participating in a clinical trial. This could include compensation for time off work or stipends that reflect the time commitment required. Where possible, research teams should choose study sites close to where their target participants live or work to further minimize travel-related expenses.
Enhance support for participants
Many clinical trials run for a long time, so it’s important to provide support to participants for the duration of the study and after its completion. Without this, participants may tire or lose interest in the study, leading to an increased dropout rate.
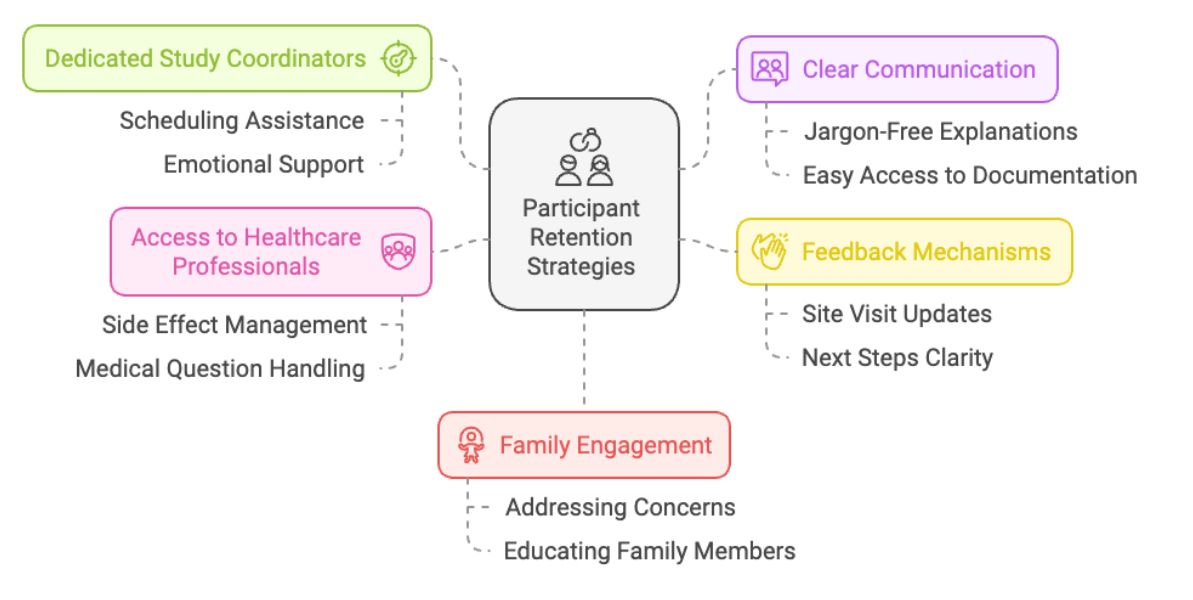
Researchers can enhance support by offering dedicated study coordinators for each participant, giving them a point of contact for scheduling, questions, and emotional support during the trial. Access to healthcare professionals is also important in managing side effects, answering medical questions, and addressing any concerns.
Clear communication is also key. Participants must fully understand the study procedures, risks, and benefits, with easy access to study documentation throughout the trial. In trials involving participants who are not fluent in the local language, translation services should be offered. This will help avoid confusion and overwhelm, while also giving the participant dedicated support in their native language where they can discuss issues.
To further enhance support, researchers should ensure participants regularly receive feedback to ensure they feel supported and involved. After each site visit, they should be informed of what is happening, with clear guidance on the next steps and what is expected from them.
An often overlooked aspect of support is family engagement. Another common reason for low retention rates is the negative influence of friends or family who are uncomfortable or unfamiliar with clinical trial procedures. Researchers should involve the participant’s family and friends in discussions to help them understand the study and bring them on board so they can support the patient at home.
Enhance participant engagement
Improving participant engagement is critical to patient retention. Without this, patients can easily lose interest in a trial and decide to leave. Research teams must provide regular updates to participants to keep them informed about the study’s progress, and where possible, provide relevant findings to help them feel engaged and valued. Participants should also feel empowered to provide feedback via surveys or regular interviews. These can help researchers gauge patient satisfaction and sentiment so they can adjust the study accordingly.
Personalize participant care
While clinical trials must stick to their protocols and offer formality where necessary, personalizing some aspects of care can help to improve patient retention rates, especially with the understanding that no two participants are ever the same. An easy way to do this is via tailored communication that acknowledges an individual’s circumstances, preferences, and motivation for joining the study.
Researchers must also ensure the study has been designed and is carried out in a way that is culturally sensitive and inclusive while respecting diverse backgrounds and needs. Participants must feel supported throughout the study with their personal views listened to by researchers.
By monitoring and predicting patient sentiment, researchers can identify areas where patients are likely to lose motivation. This allows them to address individual needs, provide better support, and improve adherence to the study, ultimately reducing dropout rates.
Leverage technology
With significant advancements in technology, researchers have an opportunity to improve the monitoring and understanding of participant experiences at every stage of a clinical trial. By utilizing emerging technology, they can improve both the trial experience and retention rates.
Wearable devices are a common method in clinical trials, with researchers able to access a continuous stream of personal health data that lets them immediately respond to any dips in patient sentiment. Various technologies can also be used to deliver appointment reminders to participants, which helps patients feel supported while ensuring they make their appointments on time.
Improve site visits
Multiple site visits can be a challenge for some participants, both in terms of their time and money, resulting in frustration and lower retention rates. The appearance and experience at the study site can also be an issue for patients. Researchers should look to improve site visits for participants, which can be done in a couple of ways.
Firstly, waiting times should be reduced out of respect for the participant’s time. Trial protocols should be well-organized and adhere to given timings throughout the duration of the study to reduce the waiting time for patients. Additionally, improving waiting room facilities can enhance the participant experience. A dedicated room with amenities like newspapers, magazines, and a television can help participants feel more comfortable and reduce boredom.
Understanding participant motivation
Using strategies to improve the patient experience during a clinical trial is critical, and understanding motivation is a key part of this. Before a trial begins, motivation is what will get a potential participant interested in joining, but it will also be what keeps them interested in the study.
A large proportion of participants are motivated to get involved because they have a chance to benefit in some way. This could be a desire to help others, a personal benefit such as improved health outcomes, or a perception that they are a good fit for the study (and vice-versa). Just as each patient is unique, their motivators will be different too. It’s important that researchers provide a range of motivational tactics to both recruit and keep patients in a study.
What motivates patients to continue participation in a clinical trial?
We know that every participant has different motivators, but there are several key motivators that most patients appreciate when participating in a clinical trial. These include:
- Minimized burden
- Clear expectation setting
- Understanding the importance of participation
- Prompt responses to inquiries
- Regular communication and timely reminders for upcoming visits
- A comfortable and patient-friendly environment
- Feeling appreciated and recognized
- Personalized care and accommodation of their schedule as much as possible
Is compensation a motivator?
Providing fair compensation to participants for costs they incur during a study is a valuable incentive, especially those with financial challenges. Compensation should always include travel to and from site visits, parking, and other incidental expenses.
However, monetary compensation should not be the sole motivator as it’s possible that unsuitable candidates will apply to take part just for monetary value, and this isn’t the goal of clinical research. It is also unlikely to be the only factor that keeps participants in late-phase clinical trials. So while inspiring and useful, compensation as a motivator has some limitations.
How to calculate clinical trial retention rates
Research teams must continually monitor and calculate clinical trial rates to measure the trial’s performance and ensure strategies can be deployed to reduce the number of dropouts. The retention rate of a trial is the percentage of enrolled participants who remain in the study and did not voluntarily leave. It can be calculated at different levels, such as the site, region, country, or overall study level.
The average retention rate for clinical trials in the USA is around 88%, which is a useful benchmark for researchers to use. However, retention rates can vary between phases and types of trials, so more specific targets must be identified in each clinical trial.
To calculate the retention rate of a study, research teams can use this formula:
- Subtract the number of subjects who voluntarily withdrew from the total number of subjects enrolled.
- Divide the result by the total number of subjects enrolled.
- Multiply the outcome by 100.
Conclusion
Patient retention in clinical trials is highly important for ensuring the successful outcome of a study. With timelines, costs, and the validity of a trial impacted by negative retention rates, it’s clear that participant retention should be front of mind for research teams when designing and carrying out a clinical trial.
In many cases, participant retention really can make or break the results of a study. The good news is that there is a range of strategies highlighted here that can be used to improve patient retention and ensure participants remain interested in a clinical trial.
