Clinical research is the driving force behind medical advancements and improving health outcomes for people all over the world. According to the World Health Organization, there were 5,999 clinical trials carried out in the USA in 2024, with thousands of participants expected to have taken part. While this sounds like a large number of trials, it’s important to remember that each trial tests something different, whether that’s a condition, treatment, or specific population.
Clinical trials can also be designed using a variety of different methodologies, with each trial type providing a series of different benefits. Research teams can choose from any one of these trial types depending on the objective of the trial and the outcome they are investigating.
Find a Clinical Trial That’s Right for You
Discover ongoing studies near you and see if you’re eligible to take part in the latest medical research. Your participation could help advance future treatments.
What is clinical research?
Clinical research is defined as the study of health, disease, and treatments involving people, tissue samples, or data. The goal of clinical research is to better understand a specific condition or treatment, supporting the wider medical community to improve their knowledge of human health and biology.
By conducting clinical trials, researchers can not only deepen their understanding of medical conditions, but can also help develop new therapies and evaluate the safety and efficacy of interventions such as drugs, devices, or other treatments. In the long term, clinical trial results can influence health outcomes and uncover better methods of preventing, diagnosing, and treating conditions or diseases.
Types of clinical research
In general terms, clinical research can be categorized into two main types: interventional and observational studies. While different, each type of clinical trial is essential for helping researchers understand health conditions and develop effective and safe treatments.
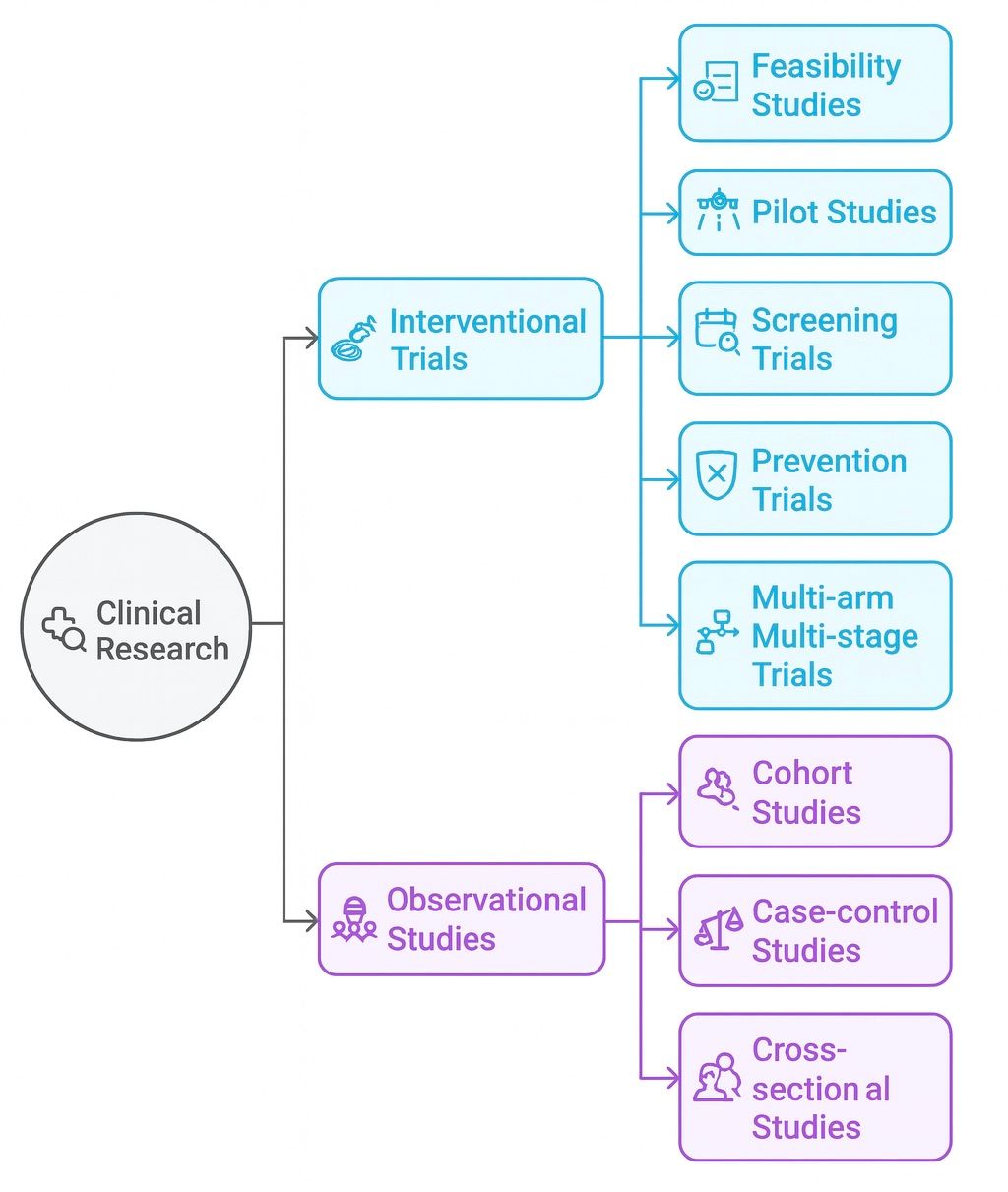
Interventional trials
Interventional trials are a type of trial that involves participants receiving one or more interventions to test their reactions. The purpose of this type of trial is to learn more about a particular intervention or treatment, such as a new drug or therapy. In many clinical trials, participants are assigned to an intervention randomly, known as randomization, so researchers can ensure more accurate results. In most cases, participants do not choose which intervention they will receive.
Observational studies
Observational studies are designed to collect data from trial participants or analyze historical data. In these trials, participants do not receive an intervention or treatment and instead are observed by a research team to find out how they behave or how this impacts their health. In some observational studies, patient registries are used, which means researchers do not need to recruit participants for a new clinical trial.
Learn how observational studies help researchers understand health trends, risk factors, and disease development over time.
Types of interventional trials
There are several types of interventional trials, each designed for a unique purpose, from testing new treatments to improving disease prevention. These trials explore different aspects of healthcare, such as evaluating the effectiveness of new drugs, exploring alternative therapies, or identifying the best methods for disease prevention. They are categorized into five main types, each serving a distinct purpose and offering its own set of benefits.
Feasibility studies
Feasibility studies are usually conducted to establish whether a main clinical trial is possible and worth carrying out. These small-scale preliminary studies evaluate a number of factors, such as participant recruitment potential, study procedures, the acceptability of the intervention to patients and healthcare providers, and how long it is likely to collect and analyze data.
Pilot studies
In some cases of clinical research, pilot studies are carried out before a large trial to ensure all parts of the study will work together properly. These studies do not seek to answer the main research question, instead, they are similar to feasibility studies and act as more of a “trial run”.
As part of a pilot study, researchers may look into previous investigations or work to identify the correct group of participants for the main study. Sometimes the data collected in a pilot study may be used in the results of the main study.
Screening trials
Screening trials can be used in clinical studies researching a specific condition to establish whether individuals have early signs of the disease before symptoms appear. In some trials, the objective may be to discover if new tests are reliable enough to detect a particular disease early or to establish whether there is a significant benefit in detecting the disease early.
Depending on the objective of the research and the condition being studied, the participant group could be either the general population or a particular group who have been identified as having a higher risk of developing the disease in question.
Prevention trials
As the name suggests, prevention trials explore whether a particular treatment or intervention can help prevent a disease from occurring or progressing. These studies may involve the general population or those at higher risk of developing the specific condition - for example, people with a family history of a certain cancer.
Multi-arm multi-stage (MAMS) trials
Multi-arm multi-stage (MAMS) trials are an innovative type of clinical trial design that allows for the simultaneous evaluation of multiple treatments within a single study. MAMS trials include the same control group throughout the study. In these studies, participants may change their treatment throughout the trial. For example, they may choose to try a new medication as it becomes available.
MAMS trials are one of the most complex types of interventional trials as they test several treatments at the same time. During the trial, researchers check how well each treatment is working and decide which ones to keep testing. In these trials, researchers also have the option to stop recruiting people to a particular group if they have enough participants to examine the results or if early results show the intervention is not working as they suspected.
Types of observational studies
Observational trials can usually be split into three different types of trials, each answering a specific research question. While some focus on understanding patterns in disease, others consider the effects of specific exposures or risk factors.
Cohort studies
In a clinical trial setting, a cohort refers to a group of people being monitored. A cohort study follows this group of people over a defined period to determine whether there are risk factors for developing a condition or disease. In some cases, these studies can run for 20-30 years.
Researchers often choose to recruit participants without the disease in question. They will follow them over a prolonged period to see who develops the disease and who doesn't. Once researchers have identified those with and without the condition, they will establish whether those with the disease have anything in common.
Case-control studies
In contrast to cohort studies, case-control studies look at two groups of people both exposed to a specific risk factor. One group is called ‘cases’ and studies people with the disease, while the other group does not have the disease and is called ‘controls’. An example of a case-control study might look at whether certain sunscreens made a difference to lifeguards and their cancer risk.
While quicker and cheaper to complete than cohort studies, case-control studies are not always accurate since they rely on participants remembering whether they were exposed to the defined risk.
Cross-sectional studies
Cross-sectional studies are done at a specific point in time or over a short period. They look at who has been exposed to a certain risk factor and who has a specific disease. For example, a cross-sectional study might investigate whether people with lung cancer were smokers in the past.
Like case-control studies, cross-sectional studies are quick and cost-effective to carry out, but the results are not always as reliable or useful since they rely on people’s memories and don’t take into account people who develop the disease later. In some research projects, a cross-sectional study is carried out to find a possible link before completing more thorough research with a case-control or cohort study.
Why do we need clinical trials?
Clinical trials are important for discovering new innovations in healthcare and medicine. They help researchers understand which treatments work, and which don’t, to better support the wider population. They are also used to identify the best methods for managing diseases, so patient outcomes can be improved and health issues prevented. Clinical research is important for testing the safety and efficacy of new treatments too, ensuring anything approved for public use is safe and effective.
There are a number of questions that clinical trials are designed to answer, including:
- Is the new treatment better than the standard treatment prescribed for this disease?
- Is the new treatment safe?
- Does the new treatment work in people?
- How well does the new treatment work?
- Do the benefits of the new treatment outweigh the risks?
- Does the new treatment cause side effects?
- What are the side effects?
- Are the side effects the same, better, or worse, than those of existing treatments?
Summary
Clinical trials are not a ‘one size fits all’ approach. Understanding the various types and when they are used is vital for gathering more accurate and reliable results which can improve healthcare for the masses. Researchers use both observational and interventional methodologies to answer research questions, with each offering a unique approach to medical research. Whether you’re keen to simply learn more about clinical trials, or you’d like to volunteer for a trial, being aware of clinical trial types is key.
